Try changing type="radio" on line 5 to checkbox.
The open-source form framework for Vue
FormKit equips developers to build their forms 10x faster by simplifying form structure, generation, validation, theming, submission, error handling, and more.
Powerful form features for data flow, error handling, and state management.
24+ accessible inputs powered by a single component.
20+ built-in validation rules and support for writing your own.
Use FormKit’s default Genesis CSS theme, Tailwind, or your own custom approach with full control over every DOM element.
Generate forms with FormKit’s JSON-compatible dynamic schema. Fully serializable for database storage.
Engineered to handle the most demanding forms.
Supported by
Introducing FormKit Themes
Open-source, MIT-licensed, Tailwind themes for FormKit.
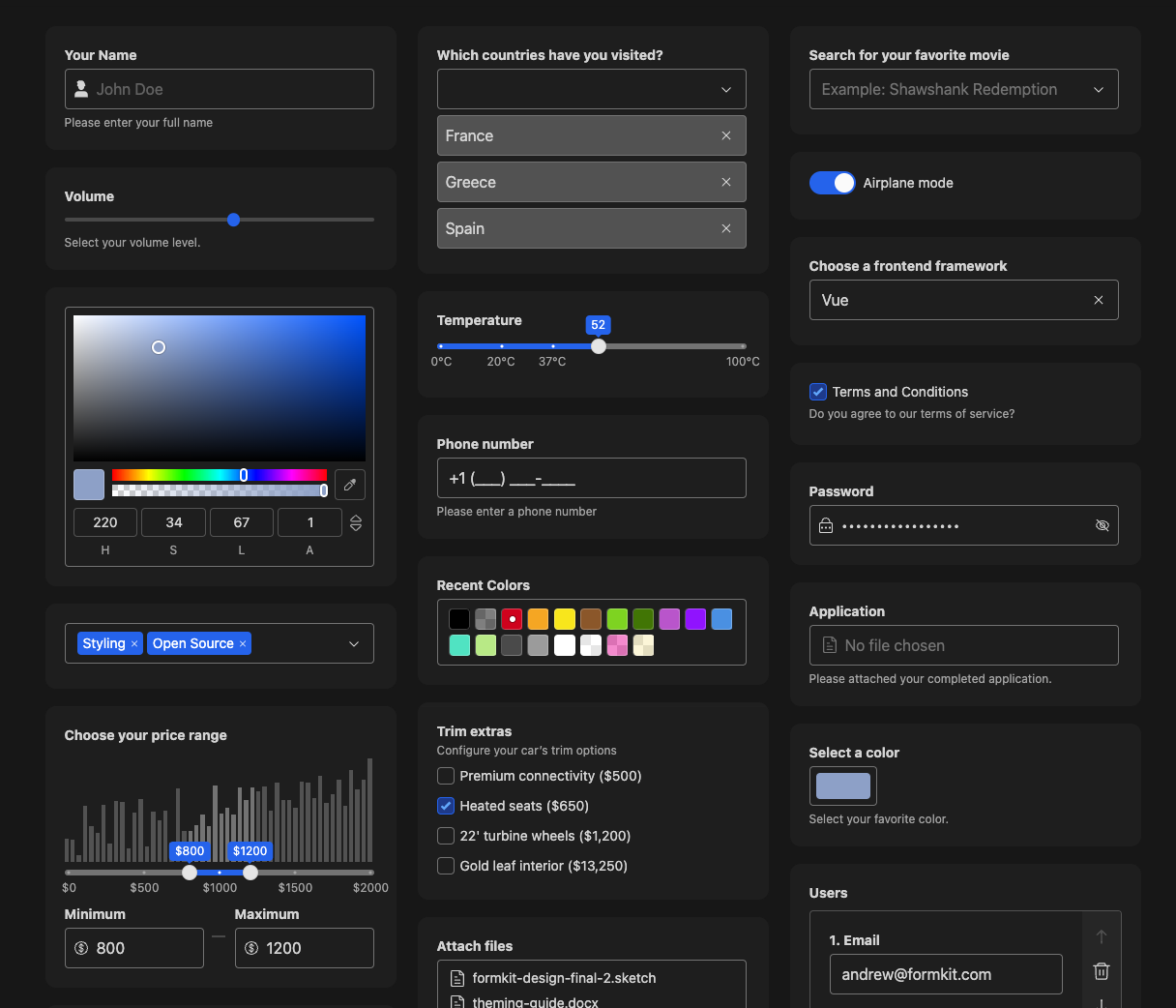
Forms
v-model Optionally v-model an entire form with one objectAutomatic loading state for async submissionsState tracking for invalid, loading, errors, and moreEasily disable all inputs in a formForm resetStructure your data as objects, arrays, and scalar valuesInputs
<FormKit />Includes every input type (24+)Accessibility built-inState tracking for invalid, loading, errors, and moreExport to restructure input HTMLCreate custom inputsControl input values with middlewareAutomatic DOM for label, help, messages, and moreSupports conditional inputsSlots for every DOM elementValidation
Styling
Schema
Try changing pricePerUser on line 2 to see the schema-powered form re-render.
Architecture
featuresExtend with hooksEvent systemWrite your own inputsFull SSR supportWrap 3rd-party inputsCLI toolCustomize with FormKit configActive community & supportInternationalization architectureHierarchical input structureSupercharge FormKit with optional Pro inputs
Powerful commercial form controls, same great API.
- Autocomplete
- Colorpicker
- Currency
- Datepicker
- Dropdown
- Mask
- Rating
- Repeaterfree
- Slider
- Taglist
- Togglefree
- Toggle Buttonsfree
- Transfer List
- Unit
Autocomplete
Search and select from a custom options list.


